Lithium-air secondary batteries (Nonaqueous Rechargeable Li-air Batteries) theoretically have an energy density of 3,505 Wh/kg or 3,436 Wh/l. If it can be successfully applied to electric vehicles, it is expected to achieve a cruising range (>500 km) comparable to that of fuel vehicles. For this reason, lithium air secondary batteries have become a research hotspot in recent years. However, previous studies have shown that the practical application of lithium-air batteries is faced with many problems and challenges.
For example, the battery has a limited number of cycles, low energy conversion efficiency, and poor rate performance. These problems have led many people to doubt the prospects for the application of lithium-air batteries and are hesitant to decide whether or not to carry out relevant research and development. Therefore, it is urgently needed to conduct research on the key issues of the practical application of lithium-air batteries, and analyze the root causes of these problems and how to obtain effective solutions to solve the above problems and truly advance the development of lithium-air batteries.
The State Key Laboratory of High Performance Ceramics and Ultrafine Structures of the Shanghai Institute of Ceramics, Chinese Academy of Sciences conducted a series of studies on the practical application of lithium-air secondary batteries.
(1) In view of the cycle life of the battery, the vertical orientation of carbon nanotubes was first used as an air electrode to visualize the evolution of the key reaction product Li2O2 for nucleation, growth, and charge-dissolution evolution (see Figure 1). It was revealed that the reversible generation and decomposition of Li2O2 on the air electrode is the core to ensure repeated cycling of the battery, and the accumulation of carbonate caused by side reactions is a major factor in the decay of the cell cycle capacity (J. Phys. Chem. C, 117, 2013, 2623-2627; J. Power Sources 235, 2013, 251-255).
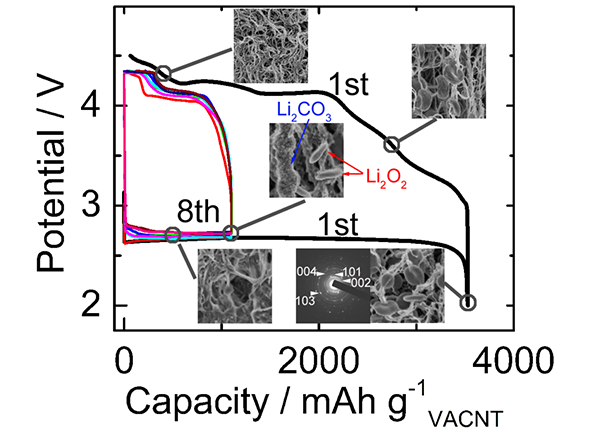
Figure 1: Evolution of surface morphology and composition of air electrodes during charging and discharging of batteries
Furthermore, by controlling the lithium-oxygen reaction depth during battery operation, Li2O2 enhanced reversibility and inhibition of formation of by-product carbonates were greatly improved, which greatly improved the cycle life of the battery (as shown in Figure 2) (Adv. Energy.) Mater., 3, 2013, 1413-1416; Energy Technol. 2, 2014, 317-324; J. Inorg. Mater., 29, 2014, 113-123).
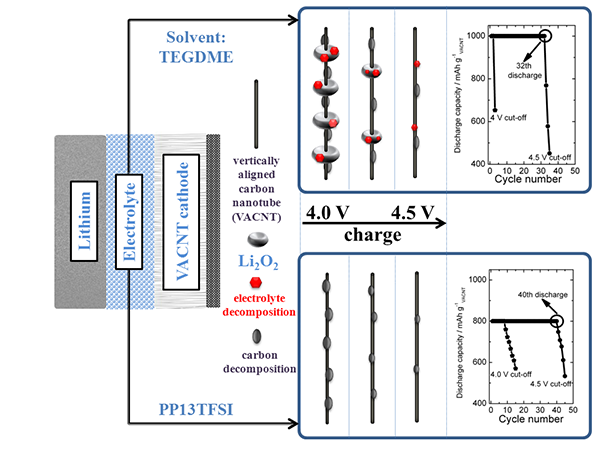
Figure 2: Effect of battery depth of discharge and charge off voltage on battery cycle life
(2) For the energy conversion efficiency of the battery, that is, there is a large voltage difference between the battery charging platform and the discharge platform (also known as the overpotential problem), the lithium oxide catalyst that has received widespread attention is first examined. Effect on energy conversion efficiency or overpotential.
It was found that although both nano-sized Au particles and nano-sized MnO particles can promote the oxygen reduction reaction, the former does not have electron exchange in the oxygen evolution reaction, but only has the effect of increasing the conductivity and promoting the decomposition of by-products, and the latter oxygen precipitation reaction at the initial cycle. There is electronic exchange. With the increase in the number of cycles, both of the Li2O2 decomposition-promoting effects are significantly attenuated due to the product-passivation of the nanoparticles (J. Phys. Chem. C, 118, 2014, 7344-7350; J. Power Sources 267, 2014, 20-25). Furthermore, the intermittent source of Galtiostatic Intermittent Titration Technique (GITT) was used to deeply analyze the origin of the overpotential in the process of battery operation (cooperating with Li Wei, a researcher at the Institute of Physics, Chinese Academy of Sciences), and the thermodynamic equilibrium was clearly indicated. In this case, the potential difference between charge and discharge can be zero (as shown in Figure 3); the thermodynamic equilibrium potential decreases with increasing temperature.
The charge and discharge polarizations are different, the former being mainly influenced by the growth kinetics of Li2O2, which is also affected by by-products (Energy. Environ. Sci. 2014, DOI: 10.1039/c4ee01777c). Interestingly, when Na is used instead of Li anode, although the product NaO2 is very similar to the Li-air battery in the discharge process, the overpotential of this system during cycling is only 0.2 V (energy conversion efficiency can reach 90%). . The main reason for this phenomenon is that NaO2 is easily decomposed during charging (Phys. Chem. Chem. Phys., 16, 2014, 15646-15652). The above research results show that the formation and decomposition of Li2O2 is the main reason for the generation of overpotential, and the easy decomposition of Li2O2 during charging will be an effective means to reduce the overpotential and improve the energy conversion efficiency of the battery.
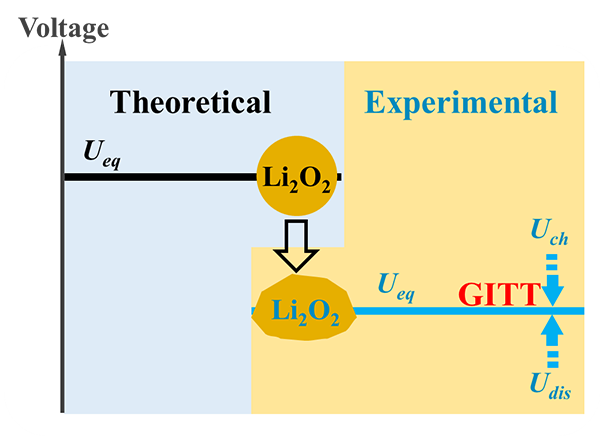
Figure 3: Comparison of the experimental and theoretical values ​​of the thermodynamic equilibrium potential of a lithium-air battery and the zero-value thermodynamic equilibrium overpotential
(3) The effect of Li2O2 morphology, reaction temperature and gas pressure, and the prenucleation rate of Li2O2 on the rate performance of Li2O2 was studied in view of poor battery performance. It was found that the morphology of the product was effectively controlled, the reaction temperature and pressure were increased, and prenucleation was performed. Can effectively increase the battery charge-discharge rate (related results are being organized during the publication process).
(4) A soft lithium-air battery with a capacity of 5 Ah was produced. A mass energy density of about 400 Wh/kg can be obtained, for example, from the mass before discharge of the battery. If the mass of the product after discharge is included, the value is about 340 Wh/kg. At the same time, it has been found that in the large-capacity battery module, the volume expansion of the positive electrode of the air and the protection of the Li negative electrode are all more prominent and require special attention; the cycle performance and the rate performance are particularly required to be improved.

Figure 4: Lithium-air battery prototype device
A series of studies on key issues in the practical application of lithium-air secondary batteries have deepened understanding of the bottlenecks that restrict the application of lithium-air batteries, and have obtained some effective solutions to these problems. The concept of the secondary lithium-ion battery was proposed in the 1980s, and it took about 10 years from its development to application. Today's scientific and technological research and development capabilities have greatly improved today, I believe that through the joint efforts of all parties R & D personnel, lithium air batteries are expected to be applied in 5 to 10 years (especially in consumer electronics products).
The above research work has been funded by the key deployment projects of the Chinese Academy of Sciences and the National Natural Science Foundation.
Field Fence also called Cattle Fence, Sheep Fence, Grassland Fences, we offer have innovative and firm structure, flat surface, uniform opening and good integration.
Woven type
Hinge joint knot & Fixed knot


|
Num |
Type |
Specification/Edge wire2.5mm/Inner wire 2.0mm |
|
1 |
7/150/813/50 |
102+114+127+140+152+178 |
|
2 |
8/150/813/50 |
89(75)+89+102+114+127+140+152 |
|
3 |
8/150/902/50 |
89+102+114+127+140+152+178 |
|
4 |
8/150/1016/50 |
102+114+127+140+152+178+203 |
|
5 |
8/150/1143/50 |
114+127+140+152+178+203+229 |
|
6 |
9/150/991/50 |
89(75)+89+102+114+127+140+152+178 |
|
7 |
10/150/1245/50 |
102+114+127+140+152+178+203+229 |
|
8 |
10/150/1194/50 |
89(75)+89+102+114+127+140+152+178+203 |
|
9 |
10/150/1334/50 |
89+102+114+127+140+152+178+203+229 |
|
10 |
11/150/1442/50 |
89(75)+89+102+114+127+140+152+178+203+229 |
Application
Field Fence is a kind of mesh used in cattle, goat, deer, and pig.
It is used for grassland, pastures, protection of ecological projects,
Papadopoulos grassland, forestry, highway and environment.
Field Fence
Cattle Fence,Sheep Fence,Farm Fence,Hinge Joint Field Fence
Anping Enzar Metal Products Co.,Ltd. , https://www.enzarmetal.com